Atoms can arrange themselves in regular configurations thanks to the Pauli exclusion principle
Source: Science News
Physicists have harnessed the aloofness of quantum particles to create a new type of crystal.
Some particles shun one another because they are forbidden to take on the same quantum state as their neighbors. Atoms can be so reluctant to overlap that they form a crystal-like arrangement even when they aren’t exerting any forces on one another, physicists report May 8 at arXiv.org. Called a Pauli crystal, the configuration is the result of a quantum mechanical rule called the Pauli exclusion principle.
Scientists had previously predicted the existence of Pauli crystals, but no one had observed them until now. “It just teaches us how beautiful physics is,” says quantum physicist Tilman Esslinger of ETH Zurich. The experiment reveals there are still new phenomena to be observed from a foundational principle taught in introductory physics classes. “If I wrote a textbook,” Esslinger says, “I would put that [experiment] in.”
Although the Pauli crystals themselves are based on known physics, the technique used to observe them could help scientists better understand certain mysterious states of matter, such as superconductors, materials that conduct electricity without resistance, or superfluids, which flow without friction.
Discovered by Austrian physicist Wolfgang Pauli in 1925, the Pauli exclusion principle forbids electrons within an atom from acquiring matching sets of quantum properties, such as energy and angular momentum. Physicists soon realized that the rule governs not only electrons but an entire class of particles called fermions, which in addition to electrons includes protons, neutrons and many types of atoms. As a result, fermions can repel one another without directly interacting. Whereas typical crystals form their regular arrangements thanks to electromagnetic interactions, a Pauli crystal forms only due to this repulsion.
“It’s the most simple state of matter that you can imagine,” says Selim Jochim of Heidelberg University in Germany.
Jochim and colleagues created their Pauli crystal out of lithium atoms, corralled by lasers into a two-dimensional region about a micrometer in radius. The researchers put groups of three or six atoms in that trap at a time. The atoms were too close together to directly image their positions to reveal any crystal-like structure. Instead, the team measured the atoms’ momenta by watching where the particles traveled when released. After the experiment was repeated many times, the researchers found correlations, or patterns, in the atoms’ momenta.
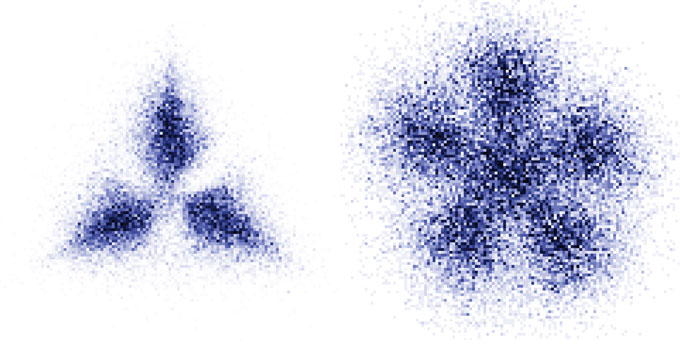
Because position and momentum are closely related properties for these trapped particles, the relationship between the momenta also means that the atoms formed a regular spatial configuration akin to a crystal. Different flower-shaped configurations of the particles’ momenta arose depending on the number of particles in the trap.
“You can really see this pattern,” says Magdalena Załuska-Kotur of the Institute of Physics of the Polish Academy of Sciences, part of a team of physicists that had previously predicted that such structures could be observed in this type of experiment.
Source: Science News




























Leave a Comment
You must be logged in to post a comment.