This is the first instance of triggering a chemical reaction with mechanical pressure alone.
Everything you’ve ever owned was shaped by chemical reactions, and with few exceptions, these chemical reactions require some type of energy in order to occur. Enzymes in your body break down and recombine atoms and molecules. Sunlight triggers fading colors, photosynthesis, and degrading plastics. Heat is perhaps the most common way to trigger chemical reactions, with fire being the most common example.
But in none of these cases is a chemical reaction triggered by simple pressure. In fact, mechanical pressure has never been used to trigger a chemical reaction before, at least until a group of researchers at the Department of Energy’s SLAC National Accelerator Laboratory and Stanford University figured out how to do it.
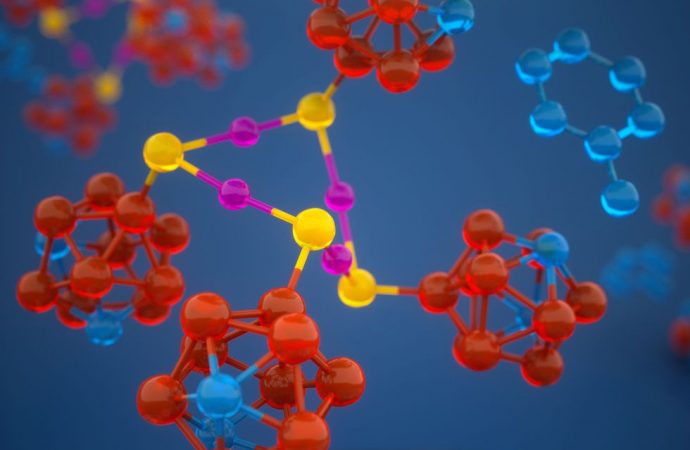
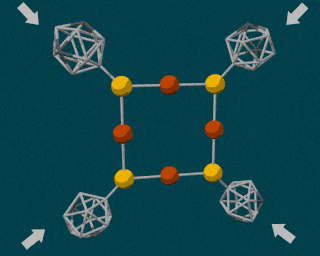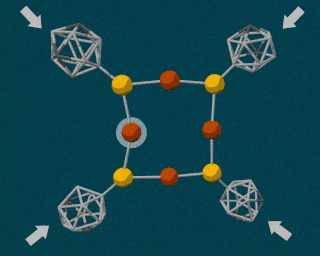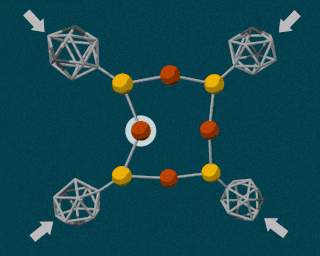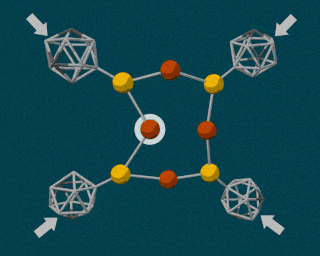
The researchers used one of the hardest substances in the world—diamonds—to produce the tremendous pressures required to break an atomic bond. Specifically, they turned to a device called a diamond anvil. This device is often used in science labs to create tremendous pressures by squeezing a substance between two flattened diamonds, and can reach pressures greater than those found at the center of the Earth.
The SLAC researchers combined the powerful diamond anvil with another durable compound called carborane. Carborane is a tiny sphere-shaped molecule made of carbon and a few other elements, and it’s capable of resisting the high pressures exerted by the diamond anvil. By attaching these tiny carboranes to a softer, squishy molecule made of copper and sulfur, the carboranes were able to act as molecular pressure points, forcing the less durable copper and sulfur atomic bonds to break apart.
Using pressure like this is certainly not the easiest way to induce a chemical reaction, so why bother? For many reactions, using pressure instead of heat is cleaner and more energy-efficient. But in a lot of cases, using this type of method is simply more precise. Using pressure, scientists can manipulate single atoms inside molecules, creating or breaking individual atomic bonds at will.
This could help plenty of other scientists who are looking for new chemicals or chemical processes, including researchers trying to develop new types of semiconductor or find a way to convert carbon dioxide into something useful. There’s still a lot of work to do, but now that we know this process exists it’s only a matter of time before it’s used in all kinds of ways.
Source: Popular Mechanics




























Leave a Comment
You must be logged in to post a comment.