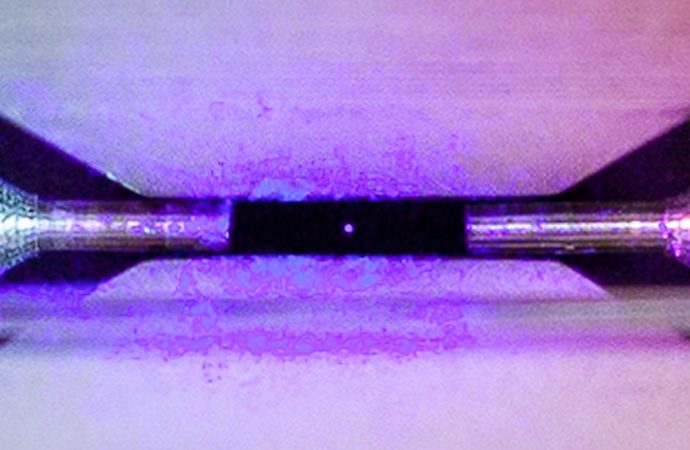
A laser is shone on the trapped strontium atom, and as it absorbs and emits energy, we can see the glow, without actually seeing the atom itself
One of the strangest things about the gorgeous photo of an atom that has just won a British science photography prize is that you cannot take a photo of an atom. It is just impossible.
And yet, there it is, a strontium atom, like a little round dot, shining clear as day. The image is called “Single Atom in an Ion Trap.”
“The idea of being able to see a single atom with the naked eye had struck me as a wonderfully direct and visceral bridge between the miniscule quantum world and our macroscopic reality,” University of Oxford physicist David Nadlinger told the U.K.’s Engineering and Physical Sciences Research Council, which awards the prize.
Nadlinger, a DPhil student, came up with the idea through his work on quantum computing.
“A back-of-the-envelope calculation showed the numbers to be on my side, and when I set off to the lab with camera and tripods one quiet Sunday afternoon, I was rewarded with this particular picture of a small, pale blue dot,” he said.
The result, obtained last August, is an image. But it is not a photo. The difference is in how the picture gets made.
The wavelength of visible light varies, from the shortest violet to the longest red, but is about 500 nanometres, give or take.
The average atom is hundreds, even thousands of times smaller than that.
The upshot is that you cannot bounce light off an atom, in the way you can bounce light off a person or a cat to capture their image.

Nadlinger’s image shows a single atom of strontium, positively charged, held in place in a vacuum by the electromagnetic field produced by two metal electrodes a mere two millimetres apart.
This device is called an ion trap, and it is a key part of research into the development of quantum computers, and in the operation of atomic clocks. In both cases, the trap is useful because it allows physicists to measure and manipulate the highly regular behaviour of atoms at the smallest scale.
At the sub-atomic scale, on the level of electrons and protons, the problem of observation goes even deeper.
Electrons, for example, are never exactly here or there. They exist in varying potentials all over the place. If you were to measure precisely where a single electron is at a given time, the wave function that describes its behaviour would collapse. In other words, you would not really be seeing the electron in all its quantum uncertainty.
What you can do, however, is make an atom glow, like a stove element on high.
In this case, a laser is shone on the strontium atom, and as it absorbs and emits energy, we can see the glow, without actually seeing the atom itself.
This image, curiously, shows the strontium atom as a rough circle in two dimensions, as if it were a sphere in real life.
This was the original conception of an atom in philosophy, going back to the ancient Greeks. The word literally means uncuttable, because atoms were thought to be the indivisible building blocks of matter.
Science has since discovered that they are, of course, nothing of the sort. Any high school student knows atoms have components of their own: a nucleus of protons and neutrons, with electrons whirling around at precise energy levels.
Mostly, though, an atom is empty space. So not only are lightwaves far too large to reflect off them, even if they were smaller, there is almost nothing to reflect off of.
Solutions to this problem have been ingenious. In 2008, American physicists used an electron microscope to capture an image of a single hydrogen atom, the smallest and lightest atom of all.
Other options include a quantum microscope, which has also been used to capture images of a hydrogen atom.
IBM has also used an “atomic force microscope” to capture images of single molecules, which are made up of many atoms in a rigid order.
Source: National Post






























Leave a Comment
You must be logged in to post a comment.