Artificial human life could soon be grown from scratch in the lab, after scientists successfully created a mammal embryo using only stem cells.
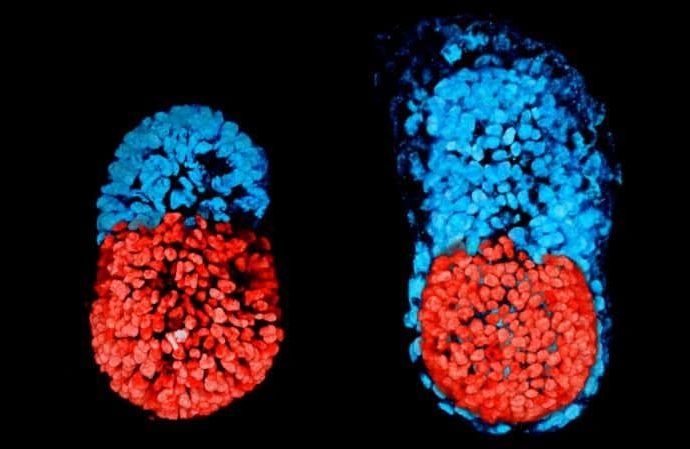
Cambridge University mixed two kinds of mouse stem cells and placed them on a 3D scaffold. After four days of growth in a tank of chemicals designed to mimic conditions inside the womb, the cells formed the structure of a living mouse embryo.
The breakthrough has been described as a ‘masterpiece’ in bioengineering, which could eventually allow scientists to grow artificial human embryos in the lab without the need for a sperm or an egg.
Growing embryos would help researchers to study the very early stages of human life so they could understand why so many pregnancies fail, but is likely to prove controversial and raise ethical questions about what constitutes human life.

Currently scientists can carry out experiments on leftover embryos from IVF treatments, but they are in short supply and must be destroyed after 14 days. Scientists say that being able to create unlimited numbers of artificial embryos in the lab could speed up research while potentially removing some of the ethical boundaries.
“We think that it will be possible to mimic a lot of the developmental events occurring before 14 days using human stem cells using a similar approach to our technique using mouse stem cells,” said Professor Magdalena Zernicka-Goetz from the Department of Physiology, Development and Neuroscience at Cambridge, who led the research.
“We are very optimistic that this will allow us to study key events of this critical stage of human development without actually having to work on (IVF) embryos. Knowing how development normally occurs will allow us to understand why it so often goes wrong.”
The embryos were created using genetically engineered stem cells coupled with extra-embryonic trophoblast stem cells (TSCs) which form the placenta in a normal pregnancy.
Previous attempts to grow embryos using only one kind of stem cell proved unsuccessful because the cells would not assemble into their correct positions.
But scientists discovered that when they added the second ‘placental’ stem cells, they two types began to talk to each other, effectively telling each other where to go.
Together they eventually melded together to form an embryonic structure, with two distinct clusters of cells at each end, and a cavity in the middle in which the embryo would continue to develop. The embryo would not grow into a mouse because it lacks the stem cells which make a yolk sack.
Britain is currently leading the world in fertility research, and last year a group at the Francis Crick Institute was granted permission to genetically modify human embryos, the first time in the world such a procedure had been approved by regulators.
However such work raises important ethical questions about the sanctity of human life and whether it should be manipulated or created in the lab at all.
Critics warn that allowing embryos to be grown for science opens the door to designer babies and genetically modified humans.
Dr David King, director of the watchdog group, Human Genetics Alert, said: “What concerns me about the possibility of artificial embryos is that this may become a route to creating GM or even cloned babies.
“Until there is an enforceable global ban on those possibilities, as we saw with mitochondrial transfer, this kind of research risks doing the scientific groundwork for entrepreneurs, who will use the technologies in countries with no regulation.”
The scientists would need to seek permission from the Human Fertility and Embryology Authority (HFEA), before attempting to create human embryos using the technique, and experts called for ‘international dialogue’ before going ahead.
Prof James Adjaye, Chair of Stem Cell Research and Regenerative Medicine, Heinrich Heine University, in Germany, said: “A regulatory body will ultimately decide on whether human stem cell embryos can be generated and for how long they can be left in the petri dish to develop further.
“Of course, there should be an international dialogue on the regulation of such experiments.”
But the study was welcomed by the scientific community who said it was a significant breakthrough.
Dr Dusko Ilic, Reader in Stem Cell Science, King’s College London, said the research was ‘masterpiece’ in creating the earliest steps of life in a lab.
“This report is significant. The group from Cambridge is actually making the embryos de novo, using two different cell types, mixing them in a specific ratio and letting them to assemble together the embryo. This is science at its best.”
The research was published in the journal Science and was funded by the Wellcome Trust and the European Research Council.
Source: The Telegraph





























Leave a Comment
You must be logged in to post a comment.